 An elderly Japanese woman who had partial vision loss due to macular degeneration of the eyes, became the first person in the world who was successfully treated with pluripotent stem cells. Although the woman’s eyesight was not much better, it seems that averted further loss of vision, which has remained stable for two years. The same, indeed, says that the vision is “brighter”.
An elderly Japanese woman who had partial vision loss due to macular degeneration of the eyes, became the first person in the world who was successfully treated with pluripotent stem cells. Although the woman’s eyesight was not much better, it seems that averted further loss of vision, which has remained stable for two years. The same, indeed, says that the vision is “brighter”.
The treatment for macular degeneration -which is slow and costly – it was launched in 2014 by Japanese researchers, headed by the ophthalmologist Μασάγιο Takahashi of the Institute of RIKEN, who have now made the relevant publication in the american medical magazine “New England Journal of Medicine” as want to mediate enough period of time to ascertain the results of treatment.
It was preceded by the oral presentation in medical conferences, and now the successful outcome was formalized with the publication.
Scientists used cells from the woman’s skin, which are reprogrammed to become pluripotent stem (iPS), and then, guided to be converted into specialized cells in the retina. The last transplanted in the eye of the patient (after previously it was surgically removed the damaged tissue), and helped in the regeneration of the retina. So, protected it from total loss of vision because of macular degeneration due to aging, the more frequent form of progressive blindness in the world.
“The group of Takahashi did an incredible job and deserve every praise. This is a landmark study that opens the door for similar treatments for many other diseases,” said one of the researchers, the leading biologist Σίνια Yamanaka, who first had discovered how to create pluripotent stem cells not from embryos but from mature somatic cells and was honored with the Nobel prize for Medicine in 2012 for his achievement.
Will follow clinical trials to more patients, and will make an effort to speed up the whole process. It remains, however, still unknown if this treatment will be able in the future to actually cure the macular degeneration that blinds slowly the people in the third age.
A permanent concern with this type of treatment is that the conversion of stem cells into new tissues may lead to cancerous mutations, something which has not happened in the case of this old woman. However, a clinical trial in another patient was discontinued necessarily in 2015, when it was found that the transplanted cells showed abnormal mutations.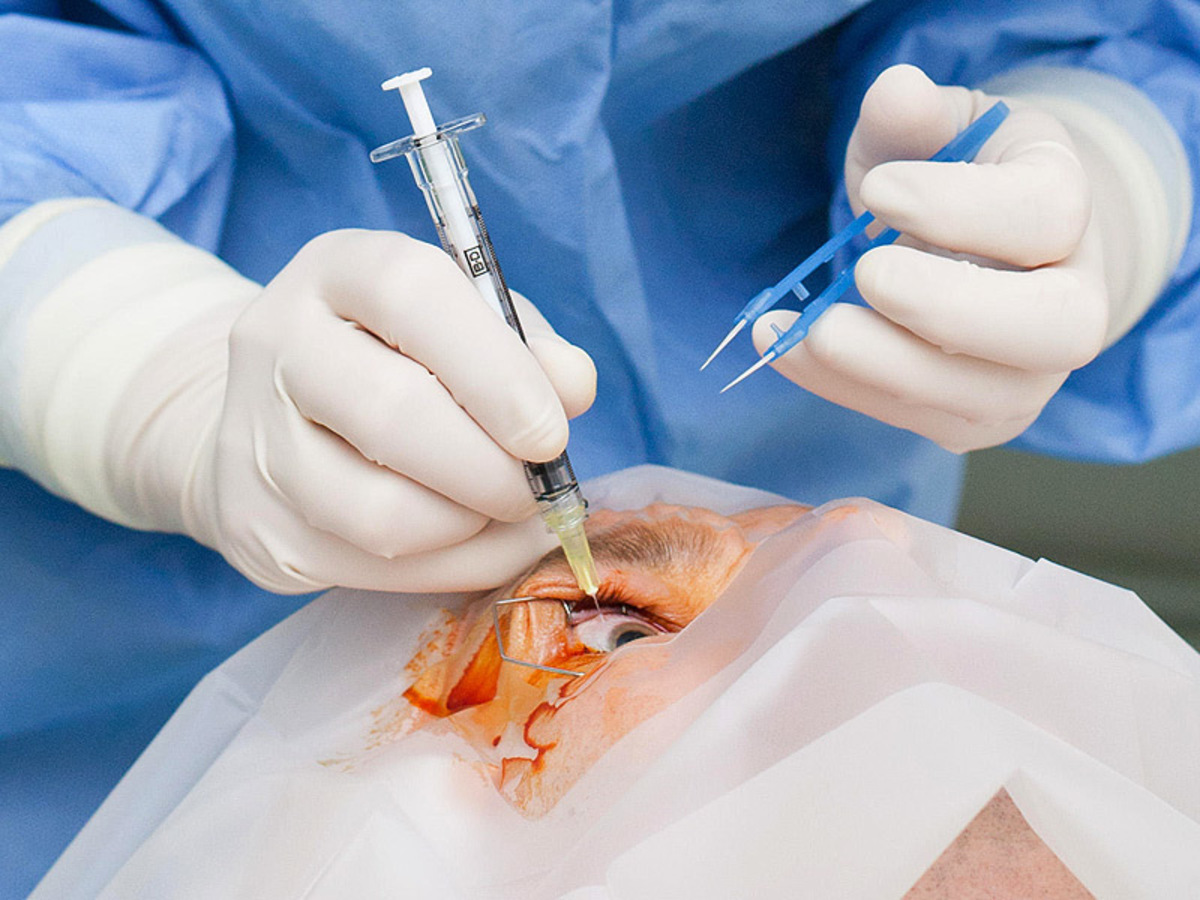
Three women were blinded
Some private medical centres have been quick to advertise a non-tested treatments with stem cells. The dangers were obvious, as well as a second study, published in the same medical journal, mentions the cases of three women 72 to 88 years of age in the united states, who made a different kind of therapy (different from the Japanese scientists) for macular degeneration.
One woman was blinded completely, while in the other two the vision has seriously deteriorated and they were left almost blind. It is very doubtful whether their vision will improve again in the future.
Each patient had to pay $ 5,000 in a private clinic of Florida in 2015 to do in the sight of the injections of stem cells, which had been taken from the fatty tissue in the abdomen. In all three patients the treatment -which had not been preceded by any scientific clinical trial – it happened at the same time in both eyes, which is contraindicated for safety reasons.
“Patients and doctors in the USA should be aware that all the clinics I βλαστοκυτταÏικών therapies’ are not safe and that the treatment provided by the american private clinics are unproven and potentially harmful,” said the associate professor of clinical ophthalmology Thomas Αλμπίνι of the University of Miami, who was called to help two of the unfortunate women.
The clinic of Florida has insisted that it has carried out thousands of βλαστοκυτταÏικÎÏ‚ treatments and the rate of complications is less than 0,01%. The researchers, however, stressed the need for a stricter surveillance mechanism of these private clinics by the competent public authorities, such as the Food and Drug administration (FDA).
From ana-MPA
Macular degeneration: Three women were blinded by controversial treatment
—